MultiLabel: top-of-the-line multi-protein expression in mammalian cells
Proteins usually are no cellular loners but interact extensively with one another in stable or transient protein complexes (see e.g. Robinson et al., 2007). Thus, while studying them in isolation still has its merits, biomedicine is moving in the direction of studying the association of proteins with one another in multi-protein complexes under different circumstances, in health and in disease (e.g. Habermann and Lange, 2012; Neto and Gould, 2011). Many processes can be studied in situ with antibodies but for certain experimental questions generating the protein complex from transgenic expression constructs is a better choice.ATG now offers MultiLabel, a suite of vectors designed specifically for multi-protein expression in mammalian cells.
| |
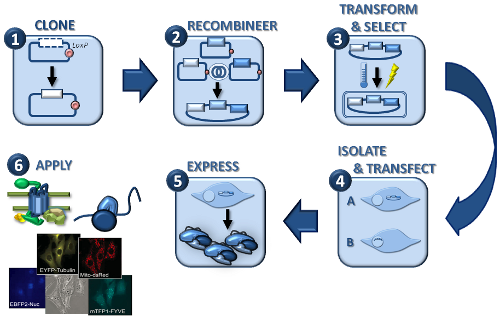
| MultiLabel workflow |
The MultiLabel system (plasmids, elements, etc.):
A short video sequence shows how cells co-express proteins carrying different fluorescent markers and how that enables tracking e.g. subcellular localization.
|
|
|
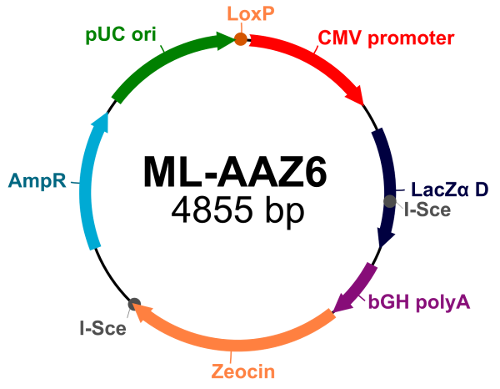
| Acceptor ML-AAZ 6 (click to expand image) |
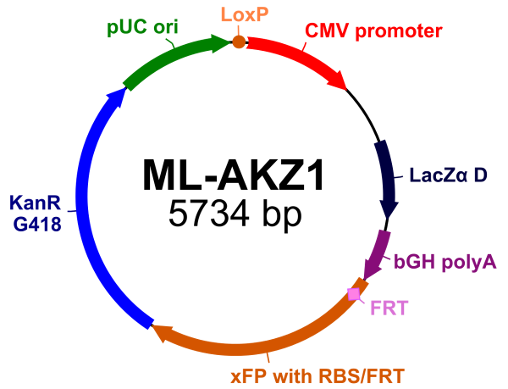
Acceptor ML-AKZ 1 (click to expand image) |
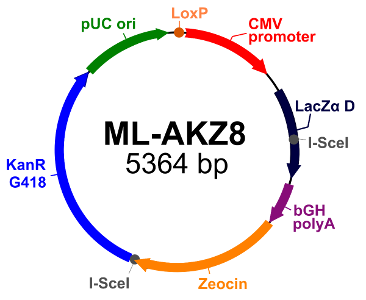
Acceptor ML-AKZ 8 (click to expand image |
|
|
|
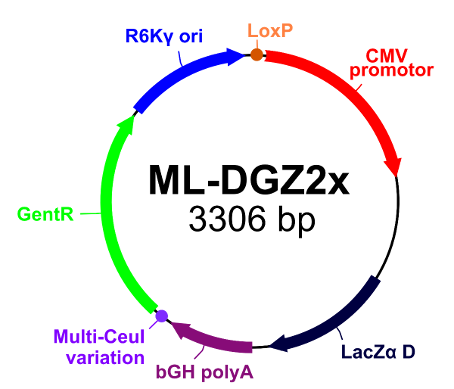
| Donor ML-DGZ2x (click to expand image) |
Donor ML-DGZ2x (click to expand image) |
A short video sequence shows how cells co-express proteins carrying different fluorescent markers and how that enables tracking e.g. subcellular localization.
Cluster Design
If you require design, optimization or adaptation of your gene cassettes for multi-gene recombineering, use ATG's expert bioinformatics service!
If you require design, optimization or adaptation of your gene cassettes for multi-gene recombineering, use ATG's expert bioinformatics service!